CAR-T (Chimeric Antigen Receptor T-cell) therapy
Why in News
Recently, NexCAR9 received approval from the Central Drugs Standard Control Organization (CDSCO) for the first chimeric antigen receptor (CAR) T-cell therapy to treat cancer, marking the first time in India.
Important Points
Background
- Cancer is a condition characterised by the uncontrolled growth and spreading of specific cells within the body. Cancer treatment primarily involves three primary modalities:
- Surgery for the removal of the cancerous tissue;
- Radiotherapy utilising ionising radiation to target the tumour;
- Systemic Therapy which entails the administration of drugs that act on the tumour.
- While surgery and radiotherapy have undergone significant refinements over time, advancements in systemic therapy have been remarkable.
- CAR T-cell therapy is a noteworthy development in this systemic therapy, currently capturing researchers’ attention worldwide.
What is CAR T-cell therapy?
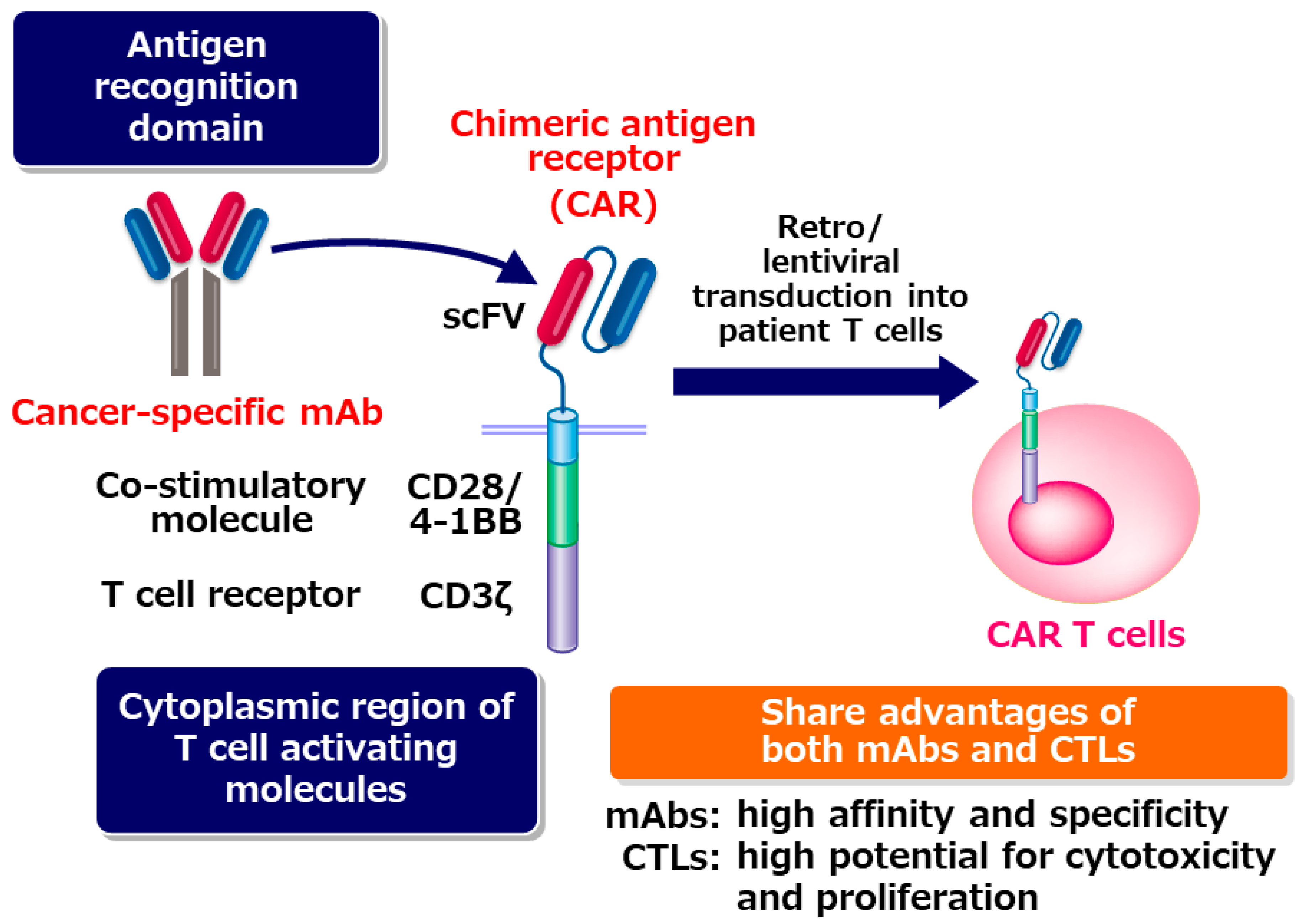
- CAR T-cell therapy is a highly advanced form of cancer treatment. Unlike chemotherapy or immunotherapy, which involve mass-produced medications, CAR T-cell therapy utilises a patient’s cells.
- These cells are modified in a lab to activate T-cells, a type of immune cell, to target and attack tumours.
- These modified cells are reintroduced into the patient’s bloodstream after being prepared to multiply more effectively.
- These cells are even more precise than targeted agents and directly stimulate the patient’s immune system to fight cancer, making the treatment exceptionally effective. They are frequently called “living drugs.“
Usage of CAR T-cell therapy
Leukaemias and Lymphomas:
- CAR T-cell therapy has been approved for treating leukaemias (cancers originating from white blood cell-producing cells) and lymphomas (from the lymphatic system).
- These cancers result from the uncontrolled growth of a single cell type, making the target for CAR T-cells consistent and dependable.
Relapse:
- CAR T-cell therapy is also used for patients whose cancers have returned after initial successful treatment or haven’t responded to previous combinations of chemotherapy or immunotherapy.
Success Rate:
- In some types of leukaemias and lymphomas, the therapy can be highly effective, with success rates as high as 90%, while in other cancer types, it is less effective.
Potential Side Effects:
- Cytokine release syndrome – a widespread activation of the immune system with collateral damage to normal cells
- Neurological symptoms – severe confusion, seizures, and speech impairment.
About NexCAR9
- Mumbai’s Immunoadoptive Cell Therapy (ImmunoACT) gained approval for India’s first CAR T-cell therapy from the Central Drugs Standard Control Organization (CDSCO).
- The treatment, called NexCAR19, focuses on CD19, a biomarker for B lymphocytes, making it a target for leukaemia immunotherapies.
- Previously, the cost of CAR-T cell therapy was exorbitant, reaching approximately $400,000 or over Rs 3.3 crore, and it was predominantly available in the United States.
- However, this breakthrough will bring the therapy within reach of patients in India. It will be offered at 20 government and private hospitals in major cities, with an estimated cost of approximately Rs 30-35 lakh per patient.
- This milestone not only offers accessible life-saving treatment in India but also extends its availability to other resource-constrained countries.
- India has joined the ranks of elite nations with access to CAR-T therapy.
Effectiveness and Unique Features
- Approximately 70% of patients respond to NexCAR19 treatment, with some achieving complete remission.
- Lab and animal studies indicate lower drug-related toxicities, including reduced neurotoxicity and Cytokine Release Syndrome (CRS).
- Trials for paediatric patients are underway at Tata Memorial Hospital, ensuring broader applicability.
Availability and Affordability
- ImmunoACT is in the process of securing licenses and partnering with hospitals, including Tata Memorial, Nanavati, Fortis, and Jaslok, across multiple cities.
- CAR-T therapy is expected to be available in a matter of weeks to a few months, pending final government approvals.
- Initially priced at Rs 30-40 lakh, ImmunoACT aims to eventually reduce the cost to Rs 10-20 lakh, making the therapy more accessible.
- Approval by regulatory agencies like CDSCO should lead to insurance coverage, but the extent may vary, and discussions with insurers and the government are ongoing.



.jpg)
.jpg)
.jpg)
.jpg)
.jpg)

.jpg)
.jpg)


